

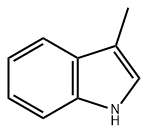
Skatole (AC)
Odour : Very strong indole-like, civet-like odour with warm and sweet aspects; a persistent odour.
Fragrance group : animal
Fragrance strength : very strong (up to 400 hours on fragrance strip at 1% dilution)
Recommended dosage: from 0.1% to 1.0%
CAS : 83-34-1
EINECS : 201-471-7
It is a complex scent that is highly dependent on concentration.
In low concentrations, skatole reveals an unexpectedly floral and sweet scent, reminiscent of the delicate nuances of jasmine and orange blossom.
However, in higher concentrations, skatole turns into a powerful and pungent odor reminiscent of the earthy and animalistic aspects of nature.
This duality makes skatole a fascinating fragrance, reflecting both the beauty and raw power of nature.
1st Class Quality Products
All fragrances are selected with care
Delivery from stock
We supply everything from our own stock, unless otherwise stated on the product itself.
Synonyms :
– 3-Methylindole
– beta-methyl indole
– 3-methyl-1H-indole
– beta-methylindole
– Scatol (Inducer)
In pure form a white powder (aged powder turns brown).
Skatole, also known as 3-methylindole, is an organic compound belonging to the indole family. It occurs naturally in the feces of mammals and birds and is the primary cause of fecal odor.
For example, skatole is used in artificial civet bases.
It is only used in minimal dosage, it takes some practice to discover the right amount. It is precisely that tiny trace of skatole that can add just the natural note needed to make a composition attractive.
Skatole is often used as a fragrance and fixative in perfumes and as a flavoring in some foods. It can also be synthesized via the Fischer indole synthesis*.
The name is derived from skato, the Greek word for manure.
* – The Fischer indole synthesis is a chemical reaction used to synthesize indoles. This reaction was first described by the German chemist Hermann Emil Fischer in 1883. The Fischer indole synthesis involves the reaction of a phenylhydrazine with a ketone or aldehyde, followed by a cyclization and elimination of water to form an indole.
Here is a simplified representation of the reaction:
Phenylhydrazine reacts with a ketone or aldehyde to form a hydrazone.
The hydrazone undergoes a cyclization to form an indole.
Water is eliminated during cyclization.
The Fischer indole synthesis is an important method in organic chemistry and is widely used for the synthesis of indole derivatives, which in turn are important building blocks for many natural products and pharmaceutical compounds.











